When scientists test a new drug in clinical trials, their purpose is to gather data that will help them assess if it is safe and effective. However, no matter their efforts, all drugs have side effects, whether they are still under investigation in clinical trials or have been authorized for use. That means that when people consume prescription drugs, over-the-counter medications, or supplements, they might present some undesirable effects. Sometimes, these effects will resolve on their own. Other times, they can be more serious and may require proper care. However, even among specialists in the area, there is often confusion regarding terms that describe these unwanted effects. In this article, we will try to decipher some of these terms.
Pharmacovigilance is the branch of science that detects, records, evaluates, and investigates undesirable events when a patient uses a medicinal product. These events might be the result of the drug or might be coincidental. Differentiating between the two cases is crucial for the safe use of medicines. Detecting a causal relationship can help scientists take measures to reduce the likelihood of occurrence of these events. They can inform healthcare providers and the public about the proper use of the drug, what errors to avoid when administering the drug, which groups of people can safely take the medication, and how to address possible harm.
But now let’s look at the often confusing terms that pharmacovigilance specialists use to describe possible unwanted events.
Adverse Event (AE)
It can be an undesired symptom, test result, or disease that a person presents after receiving a drug. However, this event is not necessarily associated with the drug that the person recently took. It might be a coincidence or caused by other factors.
Adverse Reaction (AR)
It is an adverse event, as described above. But in this case, there is a causal relationship with the drug under investigation. That means that after careful evaluation, scientists realize that the medicinal product was probably the causative agent of the reported adverse event in a particular patient.
Serious Adverse Reaction (SAR)
It is an adverse reaction that can lead to serious complications or life-threatening events.
A serious adverse reaction results in: hospitalization, prolonged stay if the patient is already in the hospital, lifelong illness or disability, congenital anomaly in case of pregnancy, or even death of the patient.
Suspected Unexpected Serious Adverse Reaction (SUSAR)
It is a reaction that fulfills the criteria of a serious adverse reaction. But, it is also an unexpected one. That means scientists did not expect this reaction to occur according to the existing data about the drug’s possible side effects.
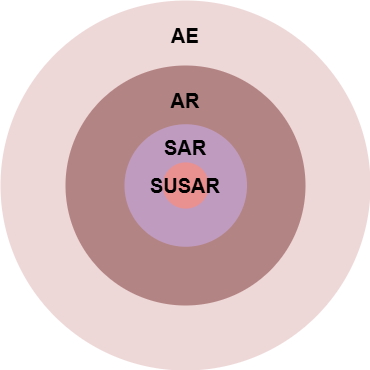
Not all adverse events are adverse reactions. If there is a possibility of a causal relationship with the drug, an adverse event is considered an adverse reaction. If the adverse reaction leads to hospitalization, death, or congenital anomalies, it is a serious adverse reaction. If a serious adverse reaction is not expected according to the existing safety information of the drug, it is termed a suspected unexpected serious adverse reaction (AE: Adverse Event, AR: Adverse Reaction, SAR: Serious Adverse Reaction, SUSAR: Suspected Unexpected Serious Adverse Reaction)
Let’s look at the following examples that describe the above terms.
- A man is prescribed a new medicine because his blood cholesterol levels are high. Two days later, he wakes up with a sore throat. The sore throat is an AE but not an AR to the new drug. Data evaluation shows that the sore throat is a symptom of a viral infection the man probably caught at a job meeting a few days earlier.
- A woman receives a vaccine against the flu. A few hours later, she has mild discomfort at the site of the injection. That is an AE attributed to the vaccine administration. Therefore, it is characterized as an AR to the vaccine.
- A boy takes penicillin to fight a bacterial infection. A few hours later, he develops a skin rash and has difficulty breathing. At the hospital, the doctors suspect this is an anaphylactic reaction. The blood tests show the boy is allergic to penicillin. Therefore, this event is considered a SAR.
Serious vs severe adverse reactions
Two other terms are usually used interchangeably, although they mean different things. Those are: a) serious and b) severe adverse reactions. As we saw above, serious adverse reactions can become life-threatening. Therefore, they refer to the possible outcome for the patient. On the other hand, the term severe adverse reaction is used to describe the intensity.
An adverse reaction can be mild, moderate, or severe. A mild AR causes a slight inconvenience, does not require treatment, or goes away shortly afterward. A moderate AR may affect daily activities, but the symptoms improve with time or minor intervention. A severe AR is debilitating. It interferes with the patient’s daily schedule, causes intolerable discomfort, and requires medical attention or treatment. However, a severe adverse reaction is not necessarily life-threatening to be considered a serious AR.
Let’s look at the following example. A headache caused by a medicinal product can be mild, moderate, or severe based on its intensity. A severe headache prevents the person from going to work or keeping up with their daily schedule. However, it will eventually go away with rest or some treatment without threatening the person’s life. A heart attack can also be mild, moderate, or severe based on symptom intensity. A heart attack, though, is considered a serious AR because it can be lethal.
The terms we discussed above can often be confusing. However, specialists in clinical trials and pharmacovigilance need to know the difference. Differentiating between cases of an adverse event is mandatory for evaluating the safety of a medicinal product and, therefore, protecting patients’ well-being.
Sources:
https://english.ccmo.nl/investigators/during-and-after-the-research/saes-and-susars