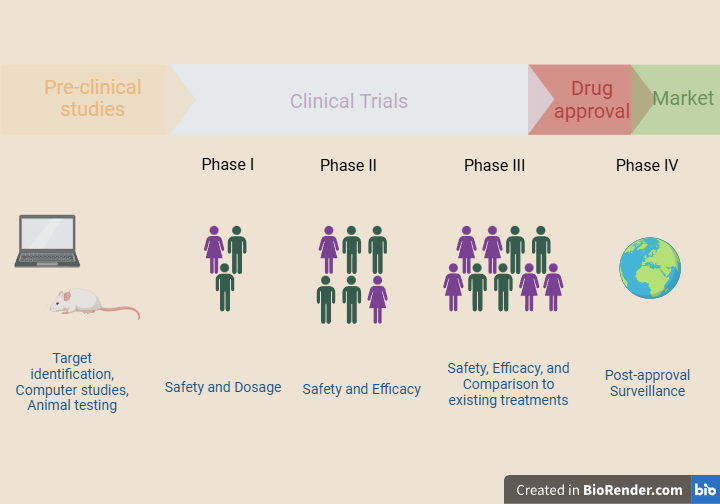
When scientists work on a new project, for example, the discovery of a new drug, it may take decades to reach the market. It often requires years of research in the lab, computer simulations, or tests on laboratory animals. Preclinical results from animal testing help scientists identify if the candidate drug is toxic or safe or if it shows any promising data regarding its effectiveness. If everything goes as planned, scientists go on to test it on human subjects in clinical trials. Therefore, clinical trials are an integral part of biomedical research.
Clinical trials study new technologies for preventing, diagnosing, or treating disease in human volunteers. In this article, we will learn about the basics of clinical trials by focusing on the ones that test new treatments.
Clinical trial design
Clinical trials are usually funded by pharmaceutical companies, national institutes, or the Ministry of Health and are carried out in hospitals, clinics, or universities. Clinical trial organizers carefully select the people to include in the study. The study participants agree to contribute voluntarily. That happens because money should not affect the decision to participate in the trial. This is important in order to protect both the human subjects’ health and the integrity of the study.
Clinical trials are overseen by regulatory bodies, such as the FDA (Food and Drug Administration) in the USA or EMA (European Medicines Agency) in Europe. The Institutional Review Board is a Bioethics committee that evaluates the available data to ensure human rights, health, and privacy protection.
Every clinical trial relies on a strict protocol with information about every step of the process. Adherence to this protocol is mandatory. Otherwise, the regulatory authorities will not allow the study to continue. The clinical trial protocol describes information about:
- The study location, the number of participants, the expected duration of the trial
- The goals of the study, the research questions that need answers, and the anticipated results
- The eligibility requirements, meaning what features the potential volunteers must have to be included in the study (e.g. age, gender, type of disease, response to previous treatments)
- The exclusion criteria, meaning what features do not allow the potential volunteers to be included in the study (e.g. presenting other comorbidities or taking certain medications)
- The tests the participants will be required to undergo during the study
- The potential benefit and the expected risk
Before the trial begins, people who consider volunteering must be thoroughly informed about the following:
- what the study involves
- what they will do during the trial
- how their health might benefit, or
- what are the possible risks
Prospective volunteers are encouraged to ask questions if there is something they do not understand. The final decision to participate in the trial is only up to them. No one will force them to participate in the study without their consent. Therefore, they have to sign a document known as an “informed consent form.” This document states that they have been fully informed about what the clinical trial involves. Also, any participant can leave the trial if they change their mind without consequences.
Phases of clinical trials
Each clinical trial consists of the following stages, known as phases:
Phase I
This part of the study involves a small group of people, usually 20-80 healthy volunteers. During this phase, researchers collect safety data and try to find the best dosage that will not be toxic to humans.
Phase II
This stage involves a few hundred people, usually 100-300 patients. It aims to determine the drug’s efficacy and further study its safety by monitoring side effects.
Phase III
Phase III involves a few thousand people, usually 1000-3000 patients. Its purpose is to study safety and efficacy in a larger group of volunteers. As more people receive the drug, it is easier to record adverse events that occur less frequently. Moreover, phase III focuses on efficacy by comparing the experimental drug to a standard treatment. This way, scientists assess whether or not the new treatment is more effective than a treatment already approved to fight the same disease. Other times, researchers compare the efficacy of the experimental drug to that of a placebo. Placebo is a substance without active ingredients. Therefore, it has no treatment value. It could, for example, be a sugar pill that is used to create a control group.
After phase III ends, the regulatory authorities will carefully review all the available data and decide if the drug is safe and effective to enter the market. They will also assess the drug’s labeling information and inspect the manufacturing sites. Sometimes, the regulatory authorities might ask the company to collect more data before they authorize the marketing of the drug.
However, even if the new drug is authorized to enter the market, its study will continue. There is a need for post-approval monitoring of side effects and efficacy, also known as phase IV of the trial. For example, it is impossible to detect rare side effects during the early phases of the clinical trials when the number of participants is small. However, as the drug starts being used by a larger population, it is easier to detect less frequent side effects. Moreover, during phase IV, scientists can study the drug’s efficacy on groups of people that were not included in clinical trials. For example, people belonging to different age groups and races or those who suffer from other comorbidities might respond differently to the new drug.
Exceptions to the standard process
Clinical trials are time-consuming. It takes years for each phase to be prepared and conducted. Then, the regulatory authorities will have to evaluate the data and decide if they will allow the study to continue. However, there are some exceptions to the standard process.
For example, as we saw in the case of the COVID-19 pandemic, the vaccines against SARS-CoV-2 were available to the public very fast. The process was rushed because there was an emergency. Otherwise, the vaccines against the virus that appeared in our lives in late 2019 would require 10-15 years to reach the market. In this case, previous research on coronaviruses and cooperation between scientists from different countries, in combination with a lot of funding, enabled the research to move very quickly. Moreover, the phases of the clinical trials overlapped. Meaning that before phase I was complete, scientists would start phase II, and so on.
Another exception to the standard clinical trial process is the study of anti-cancer treatments. Clinical studies about cancer might refer to novel surgical techniques, chemotherapeutic drugs, radiotherapy, immunotherapy, or hormonal therapy. However, due to the invasiveness and toxicity often accompanying these treatments, healthy volunteers are not included in phase I clinical trials. Instead, patients who do not fulfill the criteria to receive another drug or those who have not responded well to already-existing treatments can enter phase I clinical trials. However, as we saw earlier, phase I tests the drug’s dosage to evaluate potential toxicity. Thus, it is difficult for the drug to have a meaningful effect against the disease at this early stage of the study.
To conclude, clinical research is an extensive process. Scientists meet many obstacles during the first steps of drug discovery and development. If the drug gets to the point of human testing, clinical trials must be carefully designed. They must follow the existing regulations to protect the participants’ rights and acquire all the necessary data for the risk/benefit analysis. No matter how many in vitro experiments and animal studies show promising results in the lab, testing in humans is a crucial step to determining safety and efficacy due to the complexity of human biology. Very few drugs complete clinical trials successfully, but they are essential for advancing human health.
Sources:
https://www.nhlbi.nih.gov/research/clinical-trials/how-studies-work https://www.fda.gov/patients/clinical-trials-what-patients-need-know/basics-about-clinical-trials
https://ncirs.org.au/phases-clinical-trials