Tardigrades, also known as little water bears because of their morphology, are strange little creatures, popular among scientists trying to decipher the unique features that enable them to survive under extreme conditions. Many articles have been published regarding their genetic makeup in the hopes of using this information to treat disease or fight the consequences of radiation on health. Keep reading to find out where these fascinating abilities stem from.
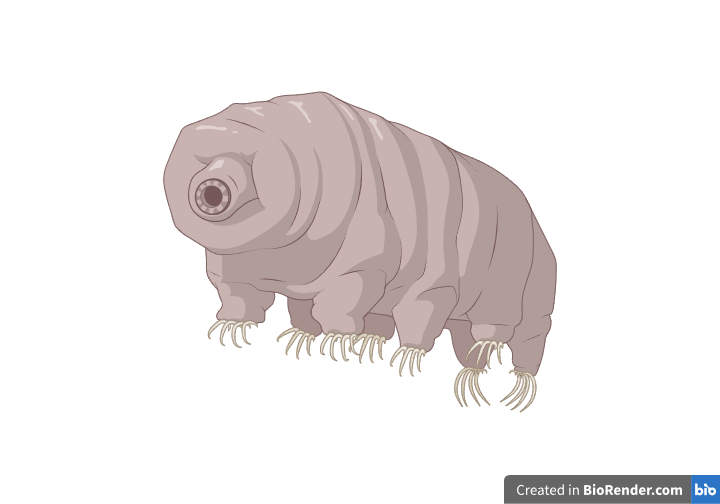
Tardigrades are little animals, not bigger than 1 millimeter in length, belonging to the phylum Tardigrada. They are similar to other invertebrates, such as arthropods, since they have four pairs of legs, they are covered by an exoskeleton, and their bodies are segmented. Their mouth, which resembles a tube, is surrounded by stylets for scratching plants and other organisms on which they are fed.

Water bears have been found in various habitats, such as terrestrial environments, fresh water, the ocean, or sandy ecosystems. However, what has caught scientists’ attention is their ability to withstand very harsh conditions, including extreme pressure at the bottom of the oceans, severely low (-273 oC) or high (150 oC) temperatures, ionizing radiation levels many times higher than what is considered lethal to humans, or even a visit to space. When tardigrades are faced with such harsh conditions, they turn into a survival mode accompanied by changes in morphology and declined metabolic rates in a process called cryptobiosis. More particularly, their bodies acquire a dried-out appearance, in which they lose lots of water, a process caused by the production of specific proteins. This transformation enables them to survive until the environmental conditions become favorable again. Tardigrades can remain in this state from a few days up to many decades, if needed, according to research findings.
Scientists have tried to understand what makes these little creatures almost indestructible by looking inside their cells and sequencing their genome. Indeed, DNA analysis has unraveled an interesting finding: tardigrades steal genes from other organisms, including bacteria, archaea, plants, and fungi. These exact genes that have been acquired from other species are believed to be responsible for their spectacular resistance to extreme stress. Researchers claim that one-sixth of their genes are foreign and have ended up inside water bears’ genome via a process known as horizontal gene transfer. But what is horizontal gene transfer?
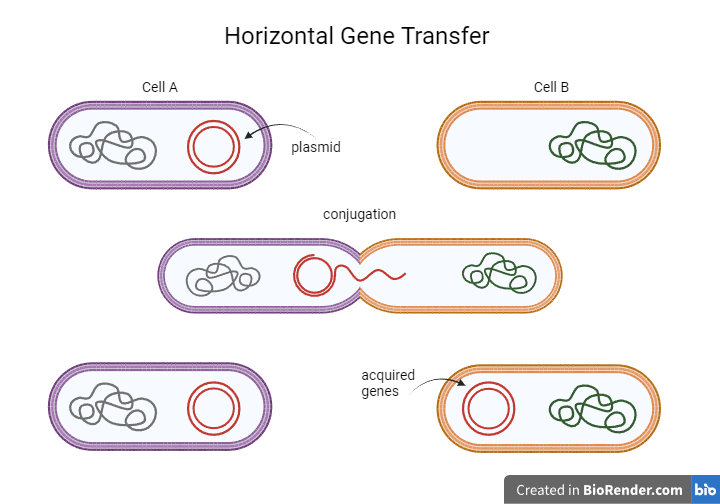
Genes usually pass from one generation to the next via traditional reproduction, less commonly known as vertical gene transfer. Instead, horizontal gene transfer occurs among different organisms less frequently. For example, it takes place between humans and viruses, where viral genes can be inserted into the human genome during an infection. Horizontal gene transfer is also quite common between bacteria, where, for example, a gene can be transferred when a donor cell is conjugated with a recipient cell during bacterial crosstalk. Throughout evolution, our interactions with other organisms have led to 1% of the human genome being made up of foreign pieces of DNA. However, tardigrades’ genome is supposed to consist of 17.5% foreign genes, a percentage higher than in any other organism studied so far.
As mentioned before, researchers have attributed the tardigrades’ gene-swapping skills to their dried-out appearance. In this situation, their cells become leaky, permitting genes and other molecules to transfer in and out of the water bear bodies. So, there is a good chance of foreign genes ending up in tardigrades’ DNA, thus giving them new abilities. On the other hand, new studies have pinpointed that the aforementioned claims about the high percentage of foreign genes might be false, as research results may have been compromised by sample contamination during laboratory analysis.
Regardless of whether horizontal gene transfer occurs at a higher rate than in other species, tardigrades can somehow avoid death in extreme conditions. That is something that might come in handy in our effort to make people’s lives better. More and more studies have discovered that tardigrades are efficient in repairing their DNA, an ability that is quite promising for researchers working in the fields of DNA damage, oxidative stress, and cancer. One of the proteins playing a major role in tardigrades’ defense against harsh environmental conditions is Dsup (Damage suppressor protein). Dsup seems to protect DNA from breaking when cells are hit by radiation, but the exact mechanism of action hasn’t been unraveled yet.
Researchers have tried to study the roles of this protein by expressing the Dsup-coding gene in cell culture, as well as animal models, such as Drosophila melanogaster (fruit fly). Some interesting findings include the Dsup protein’s ability to enter the cell nucleus, interact with DNA molecules, and decrease DNA strand breaks caused directly or indirectly by ionizing radiation. Due to its association with DNA, Dsup is believed to be also implicated in other cellular processes, e.g., chromatin remodeling and gene expression regulation.
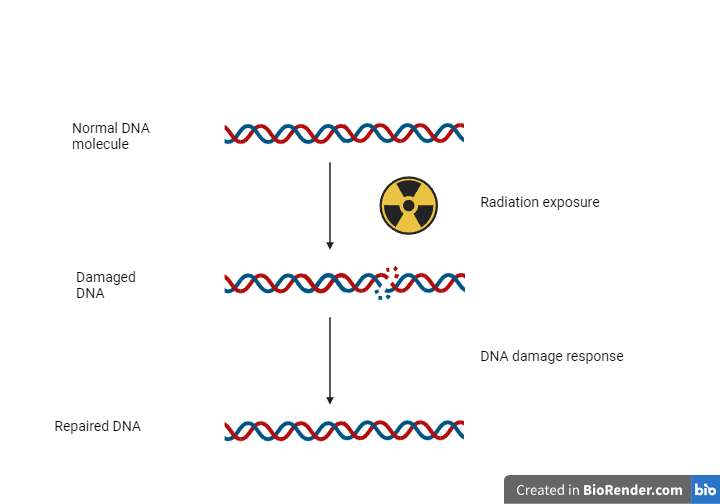
Scientists in Japan have transferred the Dsup gene from tardigrades to HEK293 cells (a human cell line commonly used in the lab) to find out if this alteration could have any effect in response to radiation. Indeed, they found out that cells containing the foreign gene presented with less DNA damage after being irradiated with X-rays in contrast to the control cells whose genome hadn’t been altered in the first place. Similar results were obtained when cells were treated with hydrogen peroxide, a substance known to cause oxidative damage to DNA.
While creating humans immune to radiation and prolonged space missions may be science fiction, at least for now, it is also a serious bioethical issue that needs proper debate. However, using genes that code for proteins that can protect our genome from radiation-induced damage could be a valuable tool in the fight against cancer, where normal cells are often unavoidably damaged during radiotherapy.
Future research can shed more light on the mechanisms that drive tardigrade cell responses, such as cell cycle arrest, DNA repair, and changes in apoptosis or autophagy pathways. This way, we can acquire new data to fight tumor cells while protecting the surrounding healthy tissues in the human body.
Sources