We have already talked about how eukaryotic and prokaryotic organisms organize their cells in order to carry out their functions in efficient ways. But how are the different cellular parts (or organelles in eukaryotes) formed and how do they carry out their functions? The answer is that everything inside a cell is the result of chemistry. That’s why cells are perceived as mini chemical factories. There are molecules that can be as small and simple as water and oxygen or large and complicated ones, as we will discuss in this article. The latter case belongs to a series of molecules called macromolecules due to their big size. Another name that is used is biomolecules since they are important molecules found in biological organisms.
What are the basic characteristics of macromolecules?
Macromolecules are divided into four categories. These are nucleic acids, proteins, lipids, and carbohydrates. Their common characteristic is that they are made up of smaller subunits, called building blocks or monomers. When the appropriate monomers bind one after the other, after the other, large chains of building blocks occur. These are the macromolecules. Since they are a series of monomers (one molecule), they are also called polymers (many molecules).
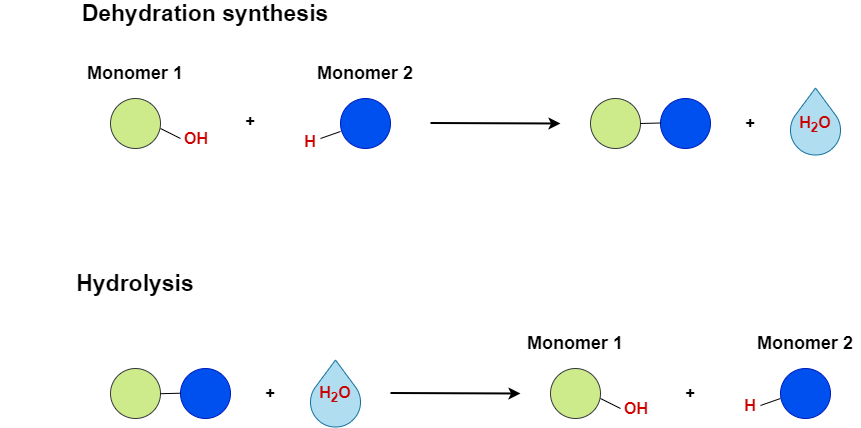
The binding of monomers is the result of a chemical reaction called dehydration synthesis. During this reaction, a hydroxyl group (OH) is released from one monomer and a hydrogen atom (H) is released from another. The OH group and the H atom form a water molecule, and that’s why the reaction is called dehydration (removal of water). The name synthesis is given because the two monomers bind, synthesizing a dimer. The bond between the monomers is a covalent bond, a very strong type of bond we can find in nature. A dimer can break apart to its monomers in a reverse chemical reaction called hydrolysis. During hydrolysis, a water molecule is added, resulting in one monomer gaining an OH group and the other gaining an H atom and, therefore, the two monomers are released intact.
Nucleic acids
There are two types of nucleic acids: deoxyribonucleic acid or DNA and ribonucleic acid or RNA. DNA is the genetic material of all living organisms and most viruses. RNA is the genetic material of some viruses, but it is also present in the cells of all the organisms of our planet carrying out very important functions. The genetic material is responsible for storing the genetic information that contains the hereditary characteristics of the cells. There are many types of RNA but the three main ones are messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA). Messenger RNA acts as an intermediate molecule between DNA and proteins and carries the information for protein synthesis. Ribosomal RNA is a basic component of the ribosome, the cellular machinery in which protein synthesis takes place. Transfer RNA carries amino acids to the site of protein synthesis so that they can be attached to the growing protein.
Structure of nucleic acids
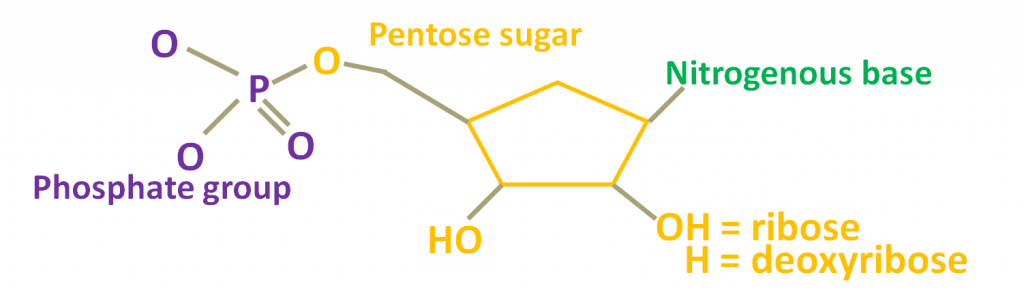
We already mentioned that nucleic acids are considered polymers (or biopolymers). This means they consist of a series of building blocks, called nucleotides that are organized in chain-like structures. DNA is a double-stranded molecule, while RNA is mostly single-stranded. Nevertheless, all strands are produced when the nucleotides are linked to one another. Each nucleotide consists of three parts: a phosphate group attached to a pentose, which in turn is also attached to a nitrogenous base. More specifically, a pentose is a five-carbon sugar that can be of two types, ribose in the case of RNA and deoxyribose in the case of DNA. There are five types of nitrogenous bases. Adenine (A) and Guanine (G), which are called purines and Thymine (T), Cytosine (C), and Uracil (U), which are also mentioned as pyrimidines. A, T, G, and C are found in DNA whereas A, U, G, and C are found in RNA.
When two successive nucleotides are connected during the dehydration synthesis reaction, a covalent bond called phosphodiester bond is formed. This is the result of a hydroxyl (OH) group leaving the first nucleotide and a hydrogen atom (H) leaving the next nucleotide and therefore, a water molecule is released. When a series of nucleotides connect the same way, one after the other, a nucleic acid strand is created. The opposite reaction occurs when a water molecule breaks the phosphodiester bond, resulting in the release of a nucleotide from the end of the strand.
Proteins
Proteins are large biomolecules that play important and various roles either inside or outside of the cells. We can divide them into a broad number of categories based on their functions. Some of them include structural proteins such as collagen, transport proteins such as hemoglobin, defensive proteins like antibodies, enzymes like amylase, and hormones such as insulin.
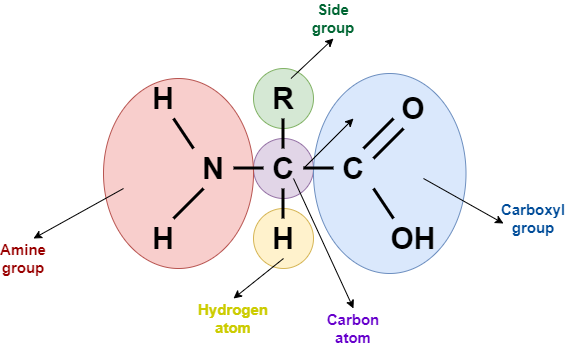
Structure of proteins
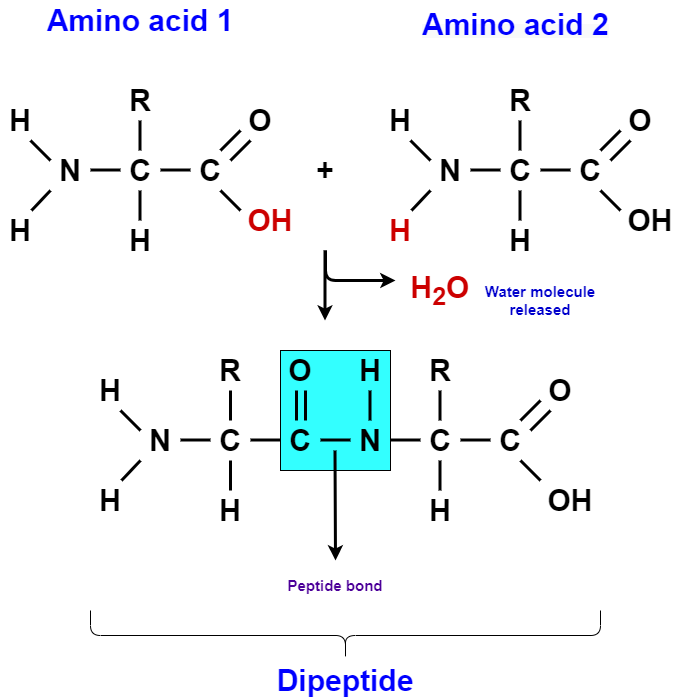
Amino acids are the building blocks of proteins. There is a large number of amino acids, but only about 20 of them occur in proteins. Each amino acid contains a carbon atom that is connected to four groups. These are an amine group (-NH2), a carboxyl group (-COOH), a hydrogen atom (H), and a side group that is symbolized as R group. The R group is unique for each amino acid and determines its properties. When two amino acids bind, they form a dipeptide, which as the name suggests, contains two amino acids. If a third amino acid is added to the dipeptide, then a tripeptide is formed. This goes on, and the result can be a polypeptide containing a large number of amino acids.
The covalent bond between two successive amino acids is called peptide bond and is again the result of a dehydration synthesis reaction. During that reaction, an OH group is removed from the COOH group of the last amino acid of the developing peptide, and an H atom is removed from the NH2 group of the amino acid that is being added. In order to break the peptide bond, a water molecule must be added in a hydrolysis reaction.
Different order of amino acids in the polypeptide chain results in a different protein. This is because of a fundamental concept in biology, saying that structure drives function. Keep in mind that the amino acid sequence determines the way the protein folds and, consequently, determines its function. Τhe sequence of the amino acids in the polypeptide comprises the primary structure. However, other interactions, called intermolecular forces, develop between certain parts of the polypeptide leading to more complex and three-dimensional structures. These are the secondary and tertiary structure. It’s also important to know that some proteins consist of more than one polypeptide chain. In this case, two or more polypeptides bind together, giving rise to the quaternary structure of the protein.
Lipids
You might come across sources claiming that lipids are not considered polymers from the entire scientific community. This is because they do not consist of a large number of monomers and, consequently, they are smaller than the other three types of macromolecules. However, lipids are one of the four basic biomolecules of cells so we will discuss them in this section. The categories of lipids we most often hear about are triglycerides, phospholipids, and steroids. You might have heard the terms fats or oils instead of triglycerides. Triglycerides are primarily stored in our adipose cells and can be used for the production of energy. Phospholipids are the basic unit of biological membranes, whether they are plasma membranes, intracellular membranes, or membranes of structures found in the extracellular environment. Their chemical structure, as we will see later, is what makes them ideal for this function. Steroids, on the other hand, have a very different structure and can be associated either with membranes or with the production of hormones.
Structure of lipids
In order to understand how most lipids are formed, we need to know a few things about fatty acids. Fatty acids are subunits of many types of lipids. They are carboxylic acids, meaning that they are long chains of carbon and hydrogen along with a carboxyl group at the end of the molecule. The bonds between the carbon atoms are mostly single covalent bonds and, therefore, the fatty acids are called saturated. There are also fatty acids with one or more double bonds, called unsaturated fatty acids. There are differences among fatty acids regarding the length of their hydrocarbon chain and the presence or absence of double bonds. Now let’s look at the structure of triglycerides. There is another subunit in addition to fatty acids. This is glycerol, a molecule consisting of three carbon atoms, each one binding to an OH group. One glycerol molecule and three fatty acids make up one triglyceride. More precisely, the glycerol’s OH groups react with the COOH groups of the fatty acids resulting in the formation of three covalent bonds, called ester bonds. Three water molecules are released in the process. The addition of three water molecules will result in a hydrolysis reaction breaking the triglyceride down to its building blocks. The three fatty acids comprising the molecule can be either the same or different. Different triglycerides consist of different fatty acids and thus have different properties.
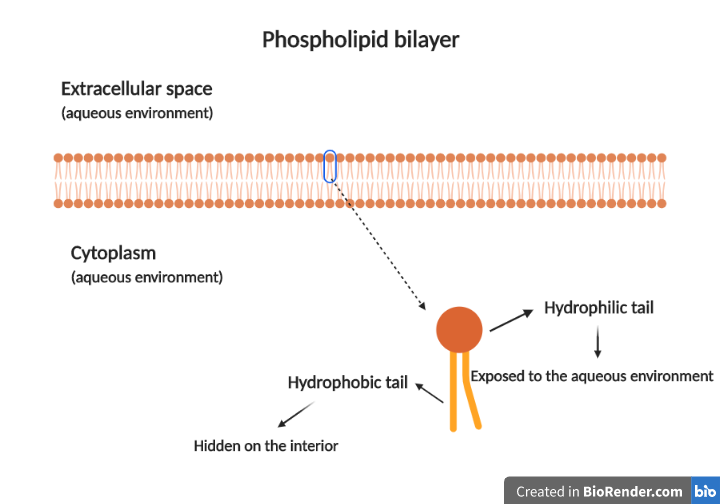
Phospholipids are a bit different from triglycerides. A glycerol molecule, in this case, binds to only two fatty acids. The glycerol’s third OH group binds to a phosphate group, which is also connected to an alcohol such as choline, serine, or ethanolamine. This structure is very important for the role that phospholipids play in living organisms. The long fatty acid chains are hydrophobic (water-fearing) due to the presence of only carbon and hydrogen atoms. This means they stay away from water. The modified phosphate group, on the other hand, is hydrophilic (likes water). This way, a phospholipid consists of a hydrophobic part, otherwise known as tail and a hydrophilic part, called head, making them amphipathic molecules. When phospholipids encounter an environment where there is water, as is the case in all living cells, they must arrange in a way that allows them to keep their hydrophobic tails away from the water. As a result, they form phospholipid bilayers. The hydrophilic heads are exposed to the aqueous environment, while the hydrophobic tails are hiding on the interior. This structure leads to the development of membranes that prevent the contents they surround from spilling out.
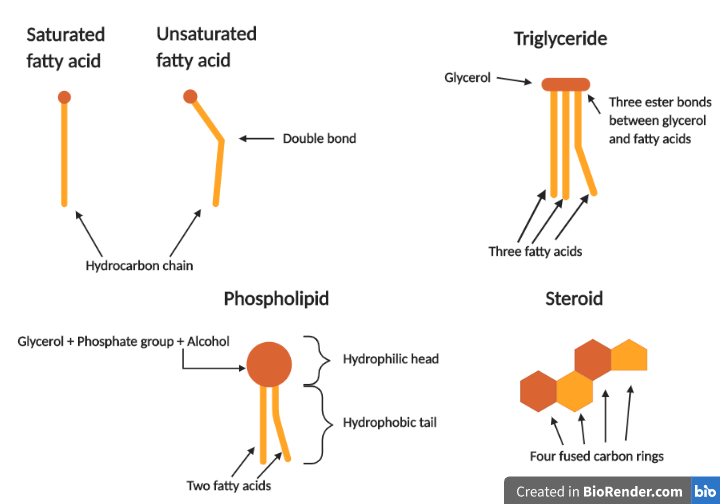
Steroids are a lot different as far as their structure is concerned. They consist of four fused carbon rings to which different chemical groups can bind, making up different steroid lipids. One steroid you might be a lot familiar with is cholesterol, which can be a part of our membranes but also plays a role in our cardiovascular health since it circulates in the bloodstream. Other examples are steroid hormones such as testosterone, estrogen, and cortisol.
Carbohydrates
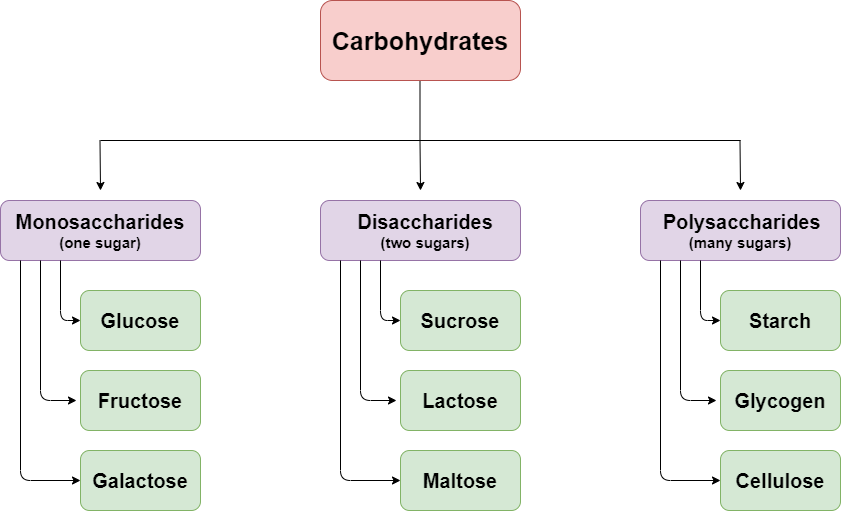
The fourth type of macromolecules we will talk about is carbohydrates. They all consist of carbon, hydrogen, and oxygen. Some carbohydrates are storage molecules since they can be used by the cell to produce energy or to release building blocks that are needed in other processes. Others have a structural role, either as small subunits that are used in the formation of complex molecules or as large polymers that provide rigidity and support. They are divided into three categories: monosaccharides, disaccharides, and polysaccharides.
Structure of carbohydrates
Monosaccharides act either on their own or as monomers for the creation of other molecules. They are classified based on the number of carbons they contain. This way, trioses have three carbon atoms, pentoses have five carbon atoms and hexoses have six. In this article, we have already come across examples of pentoses (ribose and deoxyribose), and we will also discuss some hexoses (glucose, fructose, galactose). Therefore, we understand that monosaccharides can help build not only other carbohydrates but other biomolecules such as DNA as well.
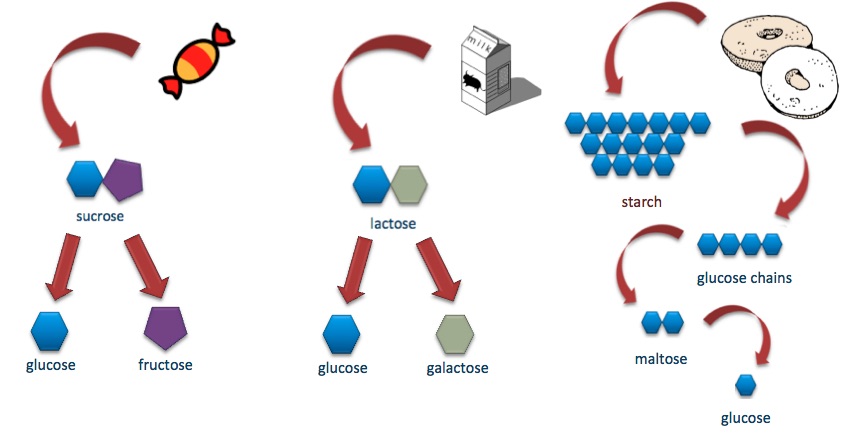
A disaccharide is formed when a glycosidic bond is formed between two monosaccharides that can be either different or the same. This is another case of a covalent bond, which results from a dehydration synthesis reaction. A hydroxyl group is released from one sugar and a hydrogen atom from the other, forming a water molecule. The disaccharides can be broken down into their subunits during a hydrolysis reaction. A few examples of disaccharides are maltose, consisting of two glucose molecules, sucrose, consisting of one glucose and one fructose molecule, and lactose, consisting of one glucose and one galactose molecule. We can find some of those and others in the food we consume. For example, lactose is a basic component of milk and yogurt, while sucrose is also known as table sugar.
The repeated binding of monosaccharides forms polysaccharides. Each one is connected to the next with a glycosidic bond. The resulting fibers can be branched or unbranched. Starch, glycogen, and cellulose are three very important polysaccharides. All three of them consist of successive glucose molecules, but the final structure and function differ among them because the monosaccharides bind in different ways. Cellulose is a structural polysaccharide that is present on all plant cells since it is the basic component of their cell wall, whose role is to provide support. Starch is another plant polysaccharide but is used by the cells to store energy in special organelles called amyloplasts. Glycogen has the same role as starch, but it is found in animal cells. When in need of energy or glucose molecules, cells can break glycogen down to make them available to the organism.