It is already known that mutations (changes in the DNA sequence) often alter the genetic information resulting in changes in the functions of cells and hence of organisms, leading to disease. So, one would expect that if a mutation is linked to a disease, it would only cause disadvantages to an individual or their offsprings. However, it has been found that some mutations are considered an advantage against other diseases. One such example is that of sickle cell anemia, a blood disorder affecting the normal functions of red blood cells and many organs of the body. That is the result of a single mutation. However, individuals carrying one copy of this mutated gene present some resistance against malaria, an infectious disease prevalent in many parts of the world.
Sickle cell anemia
Sickle cell anemia was one of the first diseases of which the genetic cause was discovered and studied extensively. Scientists found that it is the consequence of a mutation in the gene that codes for a hemoglobin chain. First, we need to discuss a few things about the structure and function of red blood cells. These are cells that travel inside the blood to transfer oxygen to the various parts of the body. That is carried out by hemoglobin, the main protein molecule of these cells. Hemoglobin consists of four polypeptide chains that fold in a certain way and interact with each other. Each one of the four chains binds a heme molecule, which is where oxygen is bound to be carried around in the body. Since the body has high oxygen demands, each red blood cell must carry as many hemoglobin molecules as possible. To achieve that, the cell gets rid of its nucleus to free up space for the hemoglobin. That is the reason behind the characteristic biconcave disc shape of red cells.
There are a few types of hemoglobin in the human body, but the main one is HbA consisting of two alpha and two beta chains. In sickle cell anemia, the sixth amino acid of the beta chain (glutamic acid) is substituted by another (valine) due to a mutation. This small change, however, affects the chain’s folding causing the hemoglobin molecule to change shape and become abnormal. Consequently, the red cell shape is altered as well, acquiring a sickle form (half-moon shape) that cannot move in the circulation effectively to transfer oxygen in the body.
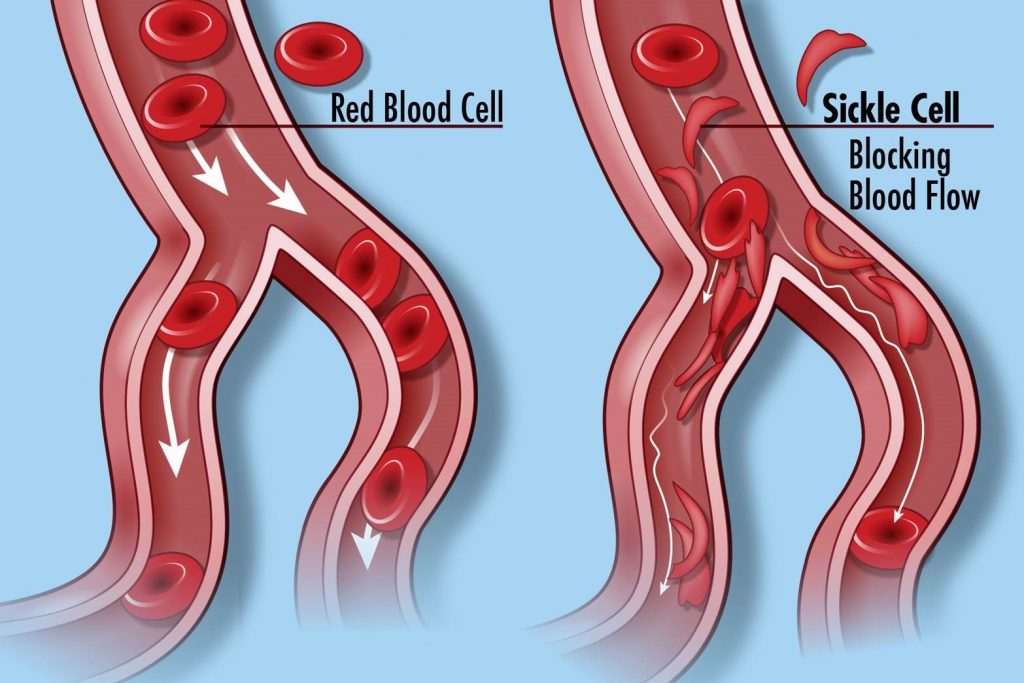
On the right: Sickle cells blocking the blood flow because of their half-moon shape.
(Image by National Human Genome Research Institute from Flickr under a Creative Commons Attribution 2.0 Generic License, no changes were made)
People with two normal copies of the beta gene possess the functional version of hemoglobin, HbA, and are symbolized as HbAA. Heterozygotes possess one normal copy and one mutated copy of the gene. They live an overall healthy life and are symbolized as HbAS. Those that have two mutated copies of the gene make only the dysfunctional hemoglobin, HbS, and are symbolized as HbSS. People belonging to the last category present severe anemia and other problems as well, such as shortness of breath, blood clotting, and reduced oxygen transfer in parts of the body. The only possible cure for this disease is a blood or bone marrow transplant, but this only applies to a small number of patients.
Malaria transmission and parasite life cycle
Malaria is an infectious disease caused by a parasite belonging to the genus Plasmodium. There are a few species that can cause this infection. The most severe form of the disease is caused by Plasmodium falciparum. People are usually infected when they are bitten by female mosquitoes of the genus Anopheles, which must have first sucked blood that is infected with the parasite. Other times, the infection is caused by sharing needles or through blood transfusions with infected blood.
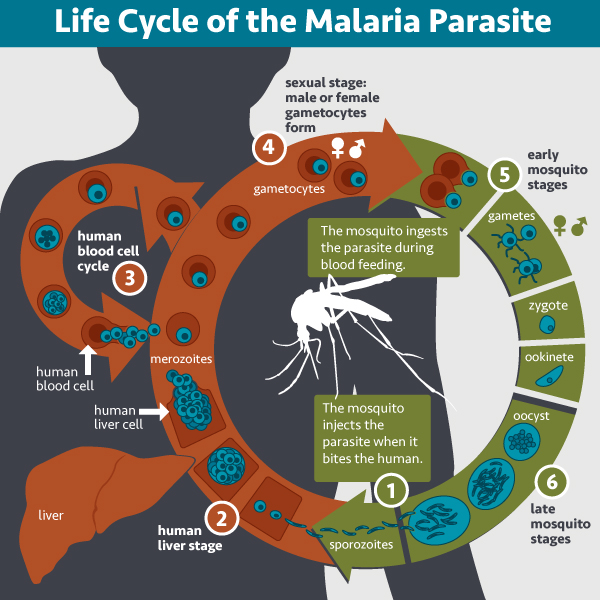
The parasite’s life cycle inside the host begins with sporozoites multiplying in liver cells to form merozoites. That is the exoerythrocytic phase (outside of red blood cells). Then, the liver cells burst, and merozoites get in the bloodstream, infecting red blood cells. That is the erythrocytic phase. That is when malaria symptoms start to occur, such as high fever, sweating, vomiting, anemia, and jaundice. The last two, in particular, are the result of the loss of red blood cells. That is caused by the parasite or by the breakdown of infected cells in the spleen. In most serious cases, malaria can be lethal.
Connection between sickle cell anemia and malaria
From early on, scientists observed that sickle cell anemia was quite common in regions of the world where malaria is prevalent, like sub-Saharan Africa. That led them to examine what was the connection between these two diseases. Soon, they discovered that the presence of the mutated gene that causes sickle cell anemia provided some resistance against malaria. That seems to be the reason why a mutation responsible for such a serious disease has been preserved in these populations by the evolutionary process.
In other words, people that are heterozygous for this mutation pose an advantage comparing to others since they do not suffer from anemia, and at the same time, they are protected against malaria. When we use the term resistance, however, we don’t mean that these individuals do not get infected at all. But if they do, they have a higher ability to fight the parasite and survive. The heterozygous people that survive will be able to reproduce and increase the presence of the mutated gene in the population.

Mechanisms of resistance
Scientists were quick to identify the connection between the two diseases. At the same time, to this day, they haven’t managed to determine the exact role this mutation plays in the protection against malaria. Many hypotheses have been made, and the research continues to identify the underlying mechanisms that explain this phenomenon.
One well-accepted suggestion is that the red blood cells of HbAS individuals create a non-favorable environment, like osmotic shrinkage, for the parasite’s growth. HbSS individuals are not protected, even though they have similar characteristics, possibly because the infection makes their anemia worse, making the situation too dangerous for survival. On the contrary, in heterozygotes, infected red blood cells are transformed to sickle cells. This way, they are easily destroyed by macrophages through phagocytosis, and the number of infected cells is reduced, alleviating the symptoms.
Another feature of infected cells is that they stick to normal red cells, creating rosette formations that obstruct blood vessels. However, this process is disturbed in heterozygotes, protecting them. That can be the result of either the formation of sickle cells or the expression reduction of some molecules adhering to the vessel walls. Research conducted in 2011 by Miguel Soares, Ana Ferreira, and colleagues of the Gulbenkian Institute of Science in Oeiras, Portugal, showed interesting results in mice. In the blood of heterozygotes, there is free heme that is absent from normal individuals and helps in the guard against the parasite. They also found that their body is used to clearing sickle cells from the bloodstream, so when the parasite attacks the red cells, it reacts immediately and fights it off. One more recent study was that of Michael Lanzer and his colleagues at Heidelberg University in Germany and the Biomedical Research Center Pietro Annigoni in Ouagadougou, Burkina Faso. Using electron microscopy, they showed that the parasite uses actin filaments of the red cell cytoskeleton of HbAA individuals to create a bridge and transfer an adherence molecule that takes part in the rosette formation and the sticking on the vessel wall. In heterozygotes, instead, this process is prevented, giving them an advantage.
It seems that a lot of effort is still required for scientists to unravel the underlying mechanisms of this protection. But more and more evidence is found with time, and we have now reached a point where the sickle cell anemia-malaria connection is more understood.
Sources: https://microbewiki.kenyon.edu/index.php/Malaria_Resistance_and_Sickle_Cell_Trait
https://www.nature.com/news/sickle-cell-mystery-solved-1.9342